The ER and Molecular Guardians of Metabolic Biology
The ER is a central organelle for protein synthesis and protein folding in the cell, and as such, is key to the maintenance of cellular homeostasis. As the demands on ER function fluctuate between cells and under different stresses, ER is equipped with adaptive responses to maintain its integrity. The most studied adaptive response system is the unfolded protein response (UPR), which senses the presence of unfolded or misfolded protein in the ER lumen through IRE1, PERK, and ATF6 branches which engage robust quality-control systems to restore equilibrium in the ER.
We are interested in new branches of ER response pathways that are specialized for metabolic adaptations. Our interest lies in understanding the mechanisms by which specialized ER-resident proteins such as Nrf1 (Nfe2L1) sense different nutrients and couple to broad adaptive programs to maintain cellular integrity and homeostasis. We also explore the interactions between inflammatory networks and ER function and the molecular mechanisms that link to metabolic homeostasis or its disruption during disease. Studies in our lab in the early 2000 demonstrated the metabolic biology of the ER and how this organelle adapts, or fails to do so, under specialized and extreme challenges, particularly in key metabolic organs. However, UPR alone cannot account for the metabolic biology of the ER and hence, we explore specialized branches of metabolic adaptive response systems in the ER.
This has recently led us to the discovery of Nrf1 (also known as Nfe2L1) in two circumstances where the canonical UPR is insufficient or even dispensable to account for the adaptation to metabolic stresses, specifically in the liver upon cholesterol overload and in brown adipose tissue upon cold exposure. Nrf1 belongs to a family of four related transcription factors and is a ubiquitously expressed ER-membrane spanning protein, belonging to the Cap’N’Collar (CNC) family of transcription factors involved in stress adaptation and detoxification. Nrf1 can sense cholesterol levels in the ER membrane through its direct binding to cholesterol and mount a defensive program to protect the liver tissue. In the absence of hepatic Nrf1, liver succumbs to massive cholesterol toxicity and inflammation. Whereas in brown adipose tissue, Nrf1 regulates proteasome activity during cold exposure to maintain the functional integrity of this tissue, offering a key role for regulated proteasome activity in metabolic homeostasis. In both BAT and WAT, absence of NRF1 triggers tissue inflammation and impairs metabolic function. We are excited for the potential of this protein to create novel therapeutic avenues in disorders with underlying ER maladaptation. In particular, we are actively investigating the molecular mechanisms of Nrf1 action in metabolic adaptation in health and how such activity can be exploited for therapeutic strategies against immunometabolic diseases.
ER Dysfunction and Inflammation
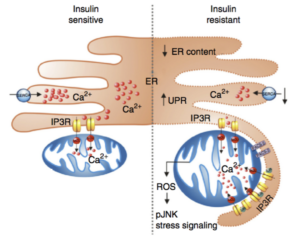 Chronic metabolic inflammation, Metaflammation, is a critical component of obesity and plays a critical role in metabolic pathologies such as insulin resistance and diabetes. During our studies exploring the mechanisms underlying the emergence of obesity-associated chronic inflammatory and stress responses such as JNK activation, we discovered abnormal ER adaptive responses and activity profiles in both experimental and human obesity. These studies suggested that ER stress and dysfunction may be proximal to emergence of metaflammation. In fact, most recently, we discovered that in adipose tissue, abnormal ER calcium homeostasis is a critical trigger for inflammation- and obesity-related JNK activation and impaired insulin action in adipose tissue and abnormal systemic glucose metabolism. These discoveries support a model that ER dysfunction and stress is a critical instigator of metaflammation.
Chronic metabolic inflammation, Metaflammation, is a critical component of obesity and plays a critical role in metabolic pathologies such as insulin resistance and diabetes. During our studies exploring the mechanisms underlying the emergence of obesity-associated chronic inflammatory and stress responses such as JNK activation, we discovered abnormal ER adaptive responses and activity profiles in both experimental and human obesity. These studies suggested that ER stress and dysfunction may be proximal to emergence of metaflammation. In fact, most recently, we discovered that in adipose tissue, abnormal ER calcium homeostasis is a critical trigger for inflammation- and obesity-related JNK activation and impaired insulin action in adipose tissue and abnormal systemic glucose metabolism. These discoveries support a model that ER dysfunction and stress is a critical instigator of metaflammation.
Interestingly however, in several different disease contexts, such as Type 1 and type 2 diabetes, we also recognized that an inflammatory environment can also impair the canonical defense systems of the ER in the liver tissue and endocrine pancreas. For example, the inflammation-induced nitrosative stress, leading to posttranslational modification of key UPR signaling molecules such as IRE1, and block its adaptive capacity. Hence, inflammation can also be proximal to ER stress and generate a vicious cycle of ER dysfunction, which also sustains metaflammation. We actively investigate the mechanisms involved in the integration of immunometabolic responses and their abnormal regulation in metabolic diseases with the goal of identifying novel strategies to manage metaflammation and Er function for therapeutic strategies and tools.
Suggested Readings:
Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002 Nov 21;420(6913):333-6. doi: 10.1038/nature01137. Abstract
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004 Oct 15;306(5695):457-61. doi: 10.1126/science.1103160. Abstract
Widenmaier SB, Snyder N, Nguyen T, Arduini A, Lee GY, Arruda AP, Saksi J, Bartelt A, Hotamışlıgil GS. NRF1 Is an ER Membrane Sensor that Is Central to Cholesterol Homeostasis. Cell. 2017 Nov; DOI:10.1016/j.cell.2017.10.003. Abstract
Bartelt A, Widenmeier SB, Schlein C, Johann K, Goncalves RLS, Eguchi K, Fischer AW, Parlakgul G, Snyder NA, Nguyen TB, Bruns OT, Franke D, Bawendi MG, Lynes MD, Leiria LO, Tseng YH, Inouye KE, Arruda AP, Hotamışlıgil GS. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasome activity. Nature Medicine. 2018 Mar; DOI: 10.1038/nm.4481. Abstract
Yang L, Calay ES, Fan J, Arduini A, Kunz RC, Gygi SP, Yalcin A, Fu S, Hotamisligil GS. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science. 2015 Jul 31;349(6247):500-6. doi: 10.1126/science.aaa0079. Abstract |Full Text
Guney E, Arruda AP, Parlakgul G, Cagampan E, Min N, Lee GY, Greene L, Tsaousidou E, Inouye K, Han MS, Davis RJ, Hotamisligil GS. Aberrant Ca2+ signaling by IP3Rs in adipocytes links inflammation to metabolic dysregulation in obesity. Sci Signal. 2021 Dec 14;14(713):eabf2059. doi: 10.1126/scisignal.abf2059. Abstract | Full Text
Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017 Feb 8;542(7640):177-185. doi: 10.1038/nature21363. PMID: 28179656.

You must be logged in to post a comment.