Role of Subcellular Molecular Architecture in Metabolic Program
Subcellular compartmentalization is a critically important dimension of metabolic activity that is not well understood. We have been interested in this critical and fundamental question and how it applies to metabolic responses under physiological fluctuations of supply and demand and metabolic pathologies. The initial leads to this program emerged from studies in liver tissue in obese animals, where we observed a marked enrichment of ER-Mitochondria interactions (MAMs). This unusual form of interaction results in abnormal calcium fluxes, mitochondrial dysfunction, oxidative stress, and metabolic impairments. These studies also suggested the possibility of dynamic regulation of the structures in healthy cells but the loss of this plasticity in the context of metabolic disease. More recently, we completed a critical study using advanced imaging technologies in large liver tissue volumes, we generated the highest resolution of subcellular structures in a native tissue environment and compared lean and obese samples under fed or fasted conditions. These studies definitively addressed the alterations in subcellular structures in obesity and upon fasting, demonstrated that molecular subcellular architecture serves as a highly dynamic regulator of metabolic homeostasis, and identified the membrane shaping proteins that are involved in this critical architectural plasticity. We have also examined the impact of feeding and fasting cycles on molecular architecture in detail and definitely addressed the nature of MAMs in physiology and obesity in very large data sets and explored other aspects of structural regulation in metabolic context. These studies also gave us an opportunity to gather a diverse set of collaborations and technologies from optics to artificial intelligence-machine learning to graphic-media arts, and from cell biology to whole animal physiology.
We are now interested in exploring other critical metabolic cells for their subcellular structural constellations and the dynamics of these features in the context of metabolic homeostasis. One of our goals is to understand the hormonal or nutritional signals that utilize structural regulation to generate their metabolic effects, and if so, through which molecular mechanisms. Finally, we explore these structural parameters in human liver tissue and their relevance to disease states.
Regulation of ER Calcium Homeostasis and Mitochondrial ROS production
While the ER is equipped with powerful adaptive programs to preserve its integrity, it succumbs to metabolic stress, such as the case in obesity. To understand the molecular mechanisms behind this maladaptive state of ER facing metabolic stress, we have systematically explored all ER resident proteins, membrane lipids, as well as ER-associated ri
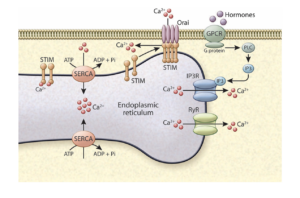
bosomes and translational profiles in highly purified ER fractions in liver tissue and compared the compositional alterations in lean and obese state. These efforts illustrated that calcium regulation is aberrant in the ER, highlighted the impact of lipogenesis and changes in the ER membrane composition of phospholipids in impairing SERCA function in the liver during obesity. Interestingly, hepatocyte Ca+2 fluxes through the IP3Rs as well as store operated calcium entry (SOCE) are also impaired in obesity contributing to the metabolic pathologies. We explore how disruption of calcium homeostasis can lead to hallmarks of obesity and diabetes: increased ER stress, metaflammation, increased glucose production, abrogated insulin sensitivity and abnormal lipid metabolism with a focus on ER and mitochondria calcium regulatory proteins during metabolic fluctuations and challenges. We hope to learn more about the metabolic effects of this versatile signaling molecules and examine potential alterations in other tissues, such as adipose tissue, that are important for our body’s metabolic mission.
Finally, we study mitochondrial dysfunction and ROS production, both as related to abnormal interorganelle communication and to the activity of the mitochondrial electron transfer chain in obesity. Most recently, we made an interesting observation that in obesity, excess ROS production in hepatic mitochondria occurs only through Complex 1 and described a mechanism for this unusual abnormal activity. We hope to utilize this knowledge to create potential novel therapeutics that involve proper regulation of organelle function in metabolic diseases.
Suggested Readings:
Arruda AP, Pers BM, Parlakgul G, Güney E, Goh T, Cagampan E, Lee GY, Goncalves RL, Hotamisligil GS. Defective STIM-mediated store operated Ca2+ entry in hepatocytes leads to metabolic dysfunction in obesity. Elife. 2017 Dec 15;6:e29968. doi: 10.7554/eLife.29968. Full Text
Fu S, Yalcin A, Lee GY, Li P, Fan J, Arruda AP, Pers BM, Yilmaz M, Eguchi K, Hotamisligil GS. Phenotypic assays identify azoramide as a small-molecule modulator of the unfolded protein response with antidiabetic activity. Sci Transl Med. 2015 Jun 17;7(292):292ra98. doi: 10.1126/scitranslmed.aaa9134. Abstract

You must be logged in to post a comment.